(E13) Where Electricity and Chemistry MeetThe link between electricity and chemistry began with a strange discovery by Luigi Galvani, a professor of anatomy in Bologna, Italy, possibly in 1786 (though he had made related observations earlier). Previous experiments with static electricity (e.g. by passing a charge through a chain of people holding hands) showed that it caused sudden muscle contractions, probably by triggering nerves which control those muscles. Galvani was dissecting frogs, and when his assistant touched a frog leg with a metal scalpel, it contracted in a similar way. Touching the frog with different metals also initiated contractions, depending on the metal, giving us today's English phrase "galvanize into action". Galvani studied the phenomenon and was convinced the electricity came from biological processes. 
Not far from Bologna, in Pavia, Alessandro Volta was professor of science. Volta had considerable experience with static electricity (in 1775 he introduced an improved version of the "electrophorus", to be discussed in a later section). Volta suggested that Galvani's electricity was not of biological origin but arose from the contact between metal and wet, salty material such as organic tissue .
Volta dispensed with the frog, experimented with metal dipped in a conducting solution, and showed that chemistry alone could produce a steady flow of electric current.
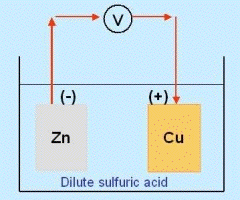
Let now a second plate be inserted into the solution, separated from the first and made of copper, and the two plates connected by an outside wire. Copper is less reactive and for simplicity, we ignore completely the interaction between it and the acid (though it does exist). The connecting wire makes the second plate, too, slightly negative relative to the fluid, so zinc ions will flow to it and will be neutralized by electrons arriving through the wire. This allows new zinc ions to enter the solution, displacing hydrogen which bubbles up near the copper. (If the second plate were also of zinc, the set-up would be symmetric, with no reason for a current to prefer flowing in either direction in the wire.) The end result is that some of the zinc ends up as zinc chloride, replacing hydrogen which bubbles up, and that an electric current flows in the wire from the copper to the zinc--the copper being electrically positive ("anode") relative to the zinc ("cathode"). The energy driving the current comes from the chemical reaction of the zinc in the solution. Many different variation of such "electric cells" have been devised using different metals, dipping either into acids or into alkalis. (Popularly they are called "electric batteries," but at least originally, "battery" meant a combination of more than one cell.) The technology of cells can be complicated. "Dry cells" used in flashlights (for instance) are not really dry--they contain liquid, too, but it is soaked into granular or fibrous material which holds it in place. Cells also contain chemicals to absorb the hydrogen H2 produced on the electrodes, whose bubbles may block the flow of current.
Compared to static electricity, which easily can reach hundreds and thousands of volts and create visible sparks (though the charge itself is small), cells typically produce about one volt, though they give a continuous flow of electric charge ("electric current"). Volta in 1800 connected a large number of cells with the (+) side of one connected by wire to the (–) side of the other, so the voltage across this entire "voltaic pile" was the sum of the contributions of all its cells. . This was the first electric battery, and was used extensively in electric experiments.
This way a storage battery may be obtained, like the ones used in motor vehicles or in portable computers. Of course, the devil is in the details: the re-deposition must restore the orderly structure of the electrodes, otherwise the storage is inefficient. Again, this calls for sophisticated (and sometimes expensive) technology: ordinary dry cells are not suitable for recharging, but rechargable cells exist using nickel and cadmium, or other metals. Automotive batteries contain liquid sulfuric acid and lead (making them quite heavy), and are nowadays sealed. It was quickly found that when electric currents from a battery are led through an ionic solution ("electrolyte"), they can produce "electrolysis", a breakdown of the chemical compound in liquid solution. Drive a current between two electrodes dipping into a solution of table salt NaCl in water: the current will split the salt ions Na+ and Cl– apart. However the experiment will not yield either sodium or chlorine. Instead the products react with the water in solution, and what comes out are hydrogen and oxygen gases, the result of splitting up the H2O molecule of water, while the sodium and chlorine recombine. Sir Humphry Davy, an early leader in the study of electrolysis, prevented this secondary reaction by heating table salt until it melted. Molten salt is also an ionic fluid, and in 1807 Davy drove a current through it and separated sodium, a soft metal which decomposed water on contact (it can be preserved immersed in kerosene). Earlier that year he decomposed potassium chloride KCl (very similar) and obtained potassium, an even more reactive soft metal which not only decomposed water but also ignited the hydrogen and oxygen thus produced. Davy made many other discoveries and was a popular lecturer, though his fame was later eclipsed by his gifted assistant and successor, Michael Faraday, who discovered the basic law of electrolysis. Expressed in modern terms, what Faraday showed was that to separate an amount of material, one always needed to pass through it a total electric charge equal to the total charge of its chemically active electrons. Look up a chemistry text for the details!
Electrolysis today is the standard method of producing aluminum, by a process invented in 1886 by Charles Martin Hall, a student at the Oberlin college in Ohio, and simultaneously by Paul Héroult in France. Prior to that aluminum was extracted using metallic potassium, a very expensive process. The Hall-Héroult process uses electrolysis of an aluminum mineral, bauxite, dissolved in a molten solution of the mineral cryolite. Because a great amount of electric energy is needed to separate aluminum, recycling it saves considerable energy.
In many cases, the circuit is not clear--some have claimed that small variations in composition have a role, also contact with the ground. Rusting is certainly more pronounced in the presence of salt water--which is why the US navy has anchored reserve ships in river estuaries, and why ships on the Great Lakes of the US tend to outlast those of the ocean. Salt spray on bridges etc. next to seawater also enhances corrosion. Stainless steel contains nickel and resists rusting by forming a tough oxide layer where it contacts the air, and aluminum resists corrosion is a similar way. Because metal corrodes, in the era of sailing, wooden hulls of ships were not joined by nails, which rust, but by wooden pegs or "trunnels" ("tree nails"). The effect is much more pronounced where two different metals come in contact. One popular way of protecting iron is by galvanizing it--dipping it in molten zinc to give it a zinc coating. A scratch in the coating will promote corrosion--but it is the more reactive zinc coating which wears away, while the iron stays protected (since the two metals have similar color, many users hardly notice the corrosion,). Only after most of the zinc is worn off does rusting suddenly accelerate. Similarly, home water heaters contain "sacrificial electrodes" of zinc (not connected to any source of electricity!) to draw corrosion away from vulnerable metal parts. An opposite effect occurs when iron is protected by coating it with tin, as is often done with preserved ("tinned") foods. Tin is less reactive than iron, so a tinned iron can looks shiny as long as the coating is intact, but scratches in it quickly tend to rust. Other examples exist, For some time, manufacturers of electric wiring for residences promoted the use of aluminum wiring. Weight for weight, aluminum wires are better conductors of electricity than copper. The problem is that the ends of wires are attached to outlets, switches and other devices, often made of copper or brass (alloy of copper and zinc). At those spots the more reactive aluminum tends to corrode and become covered by an insulating oxide layer, creating the possibility of high electric resistivity. Resistivity promotes local heating, and in rare extreme cases, that could cause fires. Remedies exist, but most home builders play it safe and continue to use copper. 
The Statue of Liberty in New York's harbor was assembled from carefully shaped sheets of copper, held in place by an iron tower. The plates were held by iron brackets, and though paint was used to separate iron from copper, over the century during which the statue has stood on Liberty Island in New York's harbor, the paint wore off and contact between iron and copper began. Luckily, copper is the less reactive of the two, so when the statue was renovated, heavily rusted iron brackets could be replaced (and insulated), while the original copper, though covered with soft green patina, was intact.
|