|
Note: Spanish translation is also available. Chemical cells, sources of the steady electrical currents in flashlights and other portable devices, were invented by Alessandro Volta in 1800. Static electricity had been known since antiquity and was studied by Coulomb, Ben Franklin and many others. Those studies, though, used relatively high voltage (aka electric potential), whereas chemical cells only produced about 1.5 volts. How did Volta know the current produced by his cells contained the same "fluid" as static electricity? In truth, I can only guess here. Volta did combine cells to form a "voltaic pile," an early electric battery, and it is possible he attained a voltage high enough to register on an electroscope. But he might also have amplified the small voltage by an instrument which he himself had invented, an electrophorus. You can easily demonstrate the second approach using mostly equipment found in the kitchen--a flat cutting board or cookie sheet, aluminum foil, saran wrap, adhesive tape, a plastic bag and a flat-bottomed frying pan. You will also need some light electrical wire (strands pulled out from a telephone cable will do), a 1.5 volt dry cell and a neon-lamp circuit tester, the kind used to check home wiring, to see whether a conductor is "hot" or not. The neon lamp needs about 70-90 volts before it lights up, but with our primitive electrophorus, the 1.5 volt cell will also produce a flash. It makes a reasonable classroom demonstration and a nice science fair project. An electrophorus is essentially a parallel plate capacitor, in which one plate can be lifted off. One metal plate lies flat on the table, on top of it a sheet of insulating material (dielectric), and on top of that, a second metal plate with an insulating handle. The capacitor is charged from some external source (e.g. a static charge produced by friction), then when the operator lifts the top plate by its handle, it is found to have a much higher voltage. Later most of the charge may be transferred to another insulated conductor, by touching it with the movable plate. By repeating the procedure again and again, that conductor can accumulate an appreciable charge, all at the higher voltage. Formally, if C is the capacitance, Q the charge on the electrophorus and V its voltage, then
where k depends on the units used. Initially the two plates are only separated by the thin dielectric, so that C is appreciable, but V is small. As the top plate is lifted, C becomes very small, but since the electric charge on top cannot escape, Q remains the same but V has to increase by the same factor as C is diminished. Hence the high voltage. On an intuitive level, initially the two plates have charges opposite signs--say + on top, - on the bottom--and these attract each other. By lifting the top plate we separate the charges and perform mechanical work against their attractive force. We have invested energy in the electric field, and it must go somewhere--in this case, it raises the voltage.
Something similar may happen with transparency sheets for a classroom projector, created on a xerox copier. If, as they emerge from the machine, you carelessly place them on top of each other, you quickly find that they firmly cling together, due to the electrical charge imparted by the copying process. If you then thoughtlessly pull them apart, the charging seems to get worse. Of course it does--by pulling the sheets apart, you have invested mechanical work and raised the voltage! Next time keep them apart, letting each cool after it emerges and allowing the charge to slowly leak away. Similarly, work against electric forces is the energy source behind the operation of the Van de Graaff generator (and other electrifying machines), as well as the mechanism which generates lightning (see http://www.phy6.org/stargaze/Svandgrf.htm).
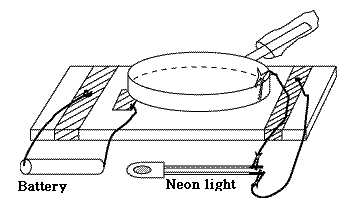 Now to our electrophorus (see drawing). The bottom plate is the flat top surface of the cutting board or cookie sheet, covered with aluminum foil; the shaded edges in the drawing are the edges of that sheet, while the middle between them is covered by another layer, drawn in white. Fold the corners of the aluminum around the bottom (as drawn) to attach the foil, or else use small pieces of tape to hold it. Over the middle of the foil wrap the saran sheet (white area under the pan), leaving on each side about one inch of aluminum exposed, for electrical connections. Wrap the pan in aluminum foil, too (not drawn; should be flat on the bottom), and slip a small plastic bag over the handle, to make sure the hand holding it is electrically insulated. Finally, tape a folded strip of aluminum to the top of the saran (see drawing), about 1.5" by 4". One end of it should be under the frying pan, the other should stick out, all on the side away from the handle. That will be your switch. You will need 4 pieces of wire. Strip the insulation about 1/2" from the ends (1" from the ends that go to the neon lamp). Pliers or a sharp knife may be used for the stripping, but just as good are a pair of nail clippers, the kind which looks like small pliers. Gently close the jaws around the place where you want to cut off the insulation, hard enough to grab the insulation but not hard enough to cut the wire--then yank it towards the end of the wire, sliding the insulation off the end. Electrically connect two wires to the ends of the dry cell, using tape to securely hold them in place. Use tape to attach the other ends of those wires--one to the bottom foil, the other to the switch. With the other two wires, wrap the longer bare ends around the terminals of the neon-light tester and secure them with tape, then tape the other ends to the bottom foil and to the foil wrapping of the frying pan. The drawing shows alligator clips connecting the wires and these work fine, too, but taping the wires to the foil or neon-light terminals is just as effective. At this point, with the pan on top of the saran, touching the "switch" foil (but nowhere in contact with the bottom foil, which would short-circuit the cell!), the frying pan is charged by the cell to 1.5 volts. The same voltage exists across the neon tube, but that of course is far too low to light it up. Now lift the pan, in such a way that it first breaks contact with the switch plate; until contact is broken, any increase in voltage is discharged through the dry cell and lost. The best way is to start the lifting by pivoting the pan around the point below the handle, so that the other end of the pan rises first. As you lift, the neon light will flash. Not a very bright flash, but unmistakable. I have also experimented with a home-made electroscope, made with aluminum foil, a drinking glass and a large paperclip, all good kitchen equipment, but beyond a tiny flutter, the voltage generated here did not register. Maybe a nice voltaic pile is needed in place of the cell... |