|
The Sun is the brightest and most familiar object in the sky. Life on Earth would not be possible without it:
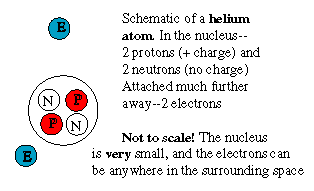
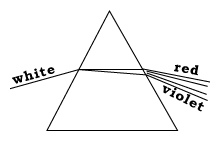
How sunlight is createdThe Sun has no sharply defined surface like that of the Earth, because it is too hot to be anything but gas. Rather, what appears to us as the surface is a layer in the Sun's atmosphere, the "photosphere" (sphere of light) which emits light ("radiates") because ot its high temperature.All hot substances radiate light, either the visible kind or beyond the rainbow spectrum, in the "infra red" (IR; "below red") and "ultra violet" (UV; "above violet") ranges. This glow [called "black body radiation" by physicists--the glow of a body with no color of its own] is the way a red-hot piece of iron or the filament in an electric light bulb produce light. The hotter the object, the brighter it shines, and the further away from red is its color. Conversely, the color of a hot object (if it is dense) tells us how hot it is. In the case of the Sun, the color of the photosphere suggests a temperature of 5780 degrees Kelvin (degrees Celsius measured from the absolute zero, about 5500° C.)
Because the ground is nowhere as hot as the Sun, its emission is also much weaker. However, at any location the ground sends out radiation in all directions in the half-sky that is visible, while receiving radiation only from the small solar disk, covering only a small circle in the sky, 0.5 degrees across. Because of this, the total energy any area receives should be equal to the total energy it returns back to space.
Think it over! If all of Earth's heat comes from the outside (neglecting internal heat), and if it maintains a steady temperature, no other way exists. Of course, only the average temperature is steady. Actually the ground is heated only in the daytime, but radiates back day and night, so nights, when energy only goes out and hardly any comes in, are cooler than days.
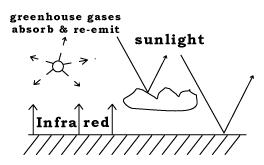
The second process is stronger, so the net effect is that like a blanket, the atmosphere helps keep Earth warmer than it would be otherwise. This is called the "greenhouse effect," because the same process operates in greenhouses used for growing vegetables in cold climates. A greenhouse is enclosed and roofed by glass panes, which let sunlight enter, but absorb the IR emitted back by the ground, and thus keep the greenhouse warm.
The chief absorbers of IR in the atmosphere are not nitrogen and oxygen, the main constituents of air, but a relatively minor percentage of "greenhouse gases" such as water vapor (H2O), carbon dioxide (CO2) and methane (CH4), which are strong absorbers of IR.
Another molecule, responsible for an important effect even though only a very small amount of it is present, is ozone, a variant of the oxygen molecule--O3 rather than the usual O2 produced at high altitudes, with its peak around 25 kilometers. It is also a greenhouse effect, but more important, it absorbs the Sun's ultra-violet (UV) light, which on can cause skin burns and hurt eyes. The ozone found near the ground and forming part of the urban air pollution comes from a completely different process.
The greenhouse effect helps keep Earth at temperatures comfortable for life, but that is a finely balanced situation. In the last half century, the burning of fossil fuels--coal and oil-- has steadily increased the atmospheric content of CO2. The average temperature of the Earth has also risen, and this rise is believed to be due to the added CO2.
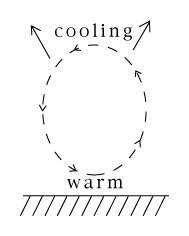
Just below the boundary of the stratosphere ("tropopause"), air which has cooled is forced down again by warmer air rising from below. The result is a circulation of air, rising hot and returning cold, going around again and again, a motion known as convection. On a cold winter day such convection also occurs in homes: near poorly insulated windows the air cools and descends (as the flame of a candle will show--but careful with that fire!), while further inside the room it rises again. The region between the ground and the stratosphere where convection and weather take place is known as the troposphere. Sunlight also evaporates water--from the oceans, from lakes and rivers and from green plants. Energy is invested in turning liquid water into vapor, and therefore humid air has more energy stored in it than dry air. The capacity of air to hold water vapor depends strongly on temperature, and is smaller in cold air (just as less sugar can dissolve in cold water). As warm humid air rises, it expands and cools, and since it then cannot hold as much water as before, the excess is forced out: Initially into the tiny droplets of clouds, then if the cooling is more drastic, into raindrops. The remaining air is drier and warmer--warmed by water vapor turning back to liquid and returning energy to its surroundings--and warmer air is better able to radiate its heat into space. That is how water, clouds and rain play a major role in the transport of solar heat from the ground back to space and help create the complex patterns of weather and climate.
Additional ExploringA very extensive master list of web sites related to weather, climate and the oceans, Wind and Sea
Two recent books discuss the history of the greenhouse effect:
|


 We look at two examples of humidity in action.
We look at two examples of humidity in action. 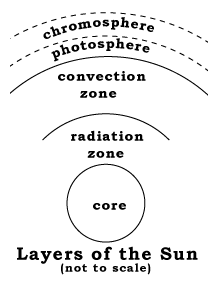
 Similarly, as one proceeds upwards from the photosphere, layers of the solar atmosphere become increasingly rarefied, and become transparent to the light coming from below them. Until the 20th century, these layers could only be observed during a total solar eclipse, when the disk of the Sun was blocked by the Moon.
Similarly, as one proceeds upwards from the photosphere, layers of the solar atmosphere become increasingly rarefied, and become transparent to the light coming from below them. Until the 20th century, these layers could only be observed during a total solar eclipse, when the disk of the Sun was blocked by the Moon.
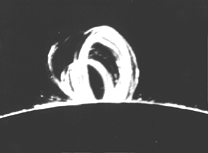 Above the middle latitudes of the Sun the streaks of the corona sometimes displayed arches, suggesting that magnetic fields there determined its structure. That impression was reinforced by "polar plumes" above the north and south poles, spread out like the iron filings at the ends of a magnet, suggesting that the Sun, like the Earth, had two magnetic poles.
Above the middle latitudes of the Sun the streaks of the corona sometimes displayed arches, suggesting that magnetic fields there determined its structure. That impression was reinforced by "polar plumes" above the north and south poles, spread out like the iron filings at the ends of a magnet, suggesting that the Sun, like the Earth, had two magnetic poles.  Galileo and Christopher Scheiner observed dark spots on the face of the Sun, and from their motion they deduced that the Sun rotated, with a period of 27 days close to the equator, relative to the moving Earth (25 days, relative to the stars). The period increased to about 29.5 days at higher latitudes, showing the Sun's surface was not solid.
Galileo and Christopher Scheiner observed dark spots on the face of the Sun, and from their motion they deduced that the Sun rotated, with a period of 27 days close to the equator, relative to the moving Earth (25 days, relative to the stars). The period increased to about 29.5 days at higher latitudes, showing the Sun's surface was not solid.  The magnetism of rare natural "lodestones" was known in ancient Greece--supposedly first noted in the town of Magnesia, from which comes the name. The magnetic compass (a Chinese discovery) was used by Columbus and other early navigators, but it was not until 1820 that a Danish professor, Hans Christian Oersted (pictured on the left), found by accident that an electric current in a wire could deflect a nearby compass needle (click
The magnetism of rare natural "lodestones" was known in ancient Greece--supposedly first noted in the town of Magnesia, from which comes the name. The magnetic compass (a Chinese discovery) was used by Columbus and other early navigators, but it was not until 1820 that a Danish professor, Hans Christian Oersted (pictured on the left), found by accident that an electric current in a wire could deflect a nearby compass needle (click 
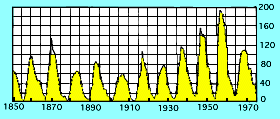
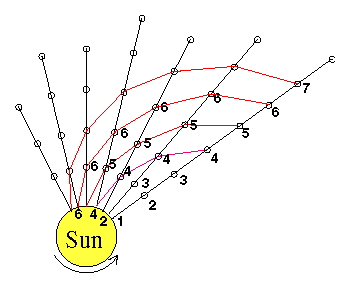



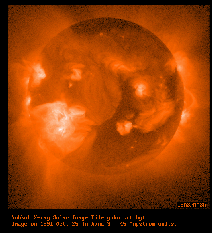 The best-known early observations of the Sun from space were the ones made in 1973 from the
The best-known early observations of the Sun from space were the ones made in 1973 from the  In one such event (picture on right),
In one such event (picture on right), 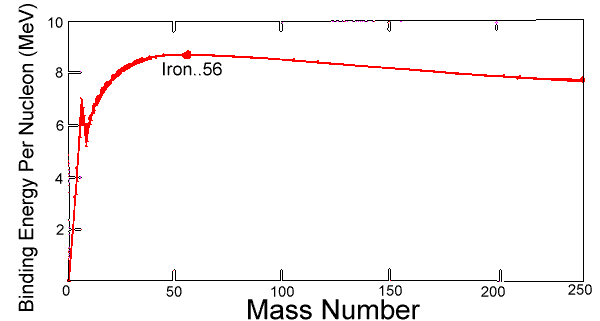
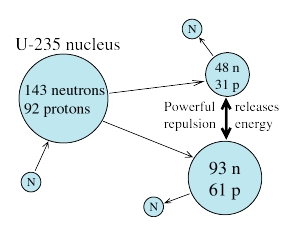
 Stars several times the size of our Sun have enough gravity to crush together not just atoms but even nuclei, compressing all their matter to a sphere perhaps 15 kilometers across. After their collapse they become "neutron stars" consisting only of neutrons (the protons all switching form), giant nuclei as dense as the ones in atoms. A huge amount of energy is liberated in that final collapse which is quite rapid, blowing off the top layers of the collapsing star and also producing elements heavier than iron.
Stars several times the size of our Sun have enough gravity to crush together not just atoms but even nuclei, compressing all their matter to a sphere perhaps 15 kilometers across. After their collapse they become "neutron stars" consisting only of neutrons (the protons all switching form), giant nuclei as dense as the ones in atoms. A huge amount of energy is liberated in that final collapse which is quite rapid, blowing off the top layers of the collapsing star and also producing elements heavier than iron. 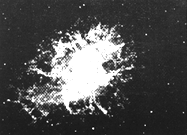 As for the "supernova remnant" left over from the collapse, its fate depends on its mass. If the star was not too massive, the remnant (as explained) is a neutron star. It that star originally rotated around its axis, that rotation is enormously speeded up; the remnant of the supernova of the year 1054 (its ejected cloud, the "Crab Nebula," is shown on the left) is spinning at about 30 revolutions per second! Any magnetic field of the original star is also enormously amplified, and associated phenomena can make it beam radio waves. Pulsars, pulsed radio sources with remarkably stable pulsation periods, are produced that way.
As for the "supernova remnant" left over from the collapse, its fate depends on its mass. If the star was not too massive, the remnant (as explained) is a neutron star. It that star originally rotated around its axis, that rotation is enormously speeded up; the remnant of the supernova of the year 1054 (its ejected cloud, the "Crab Nebula," is shown on the left) is spinning at about 30 revolutions per second! Any magnetic field of the original star is also enormously amplified, and associated phenomena can make it beam radio waves. Pulsars, pulsed radio sources with remarkably stable pulsation periods, are produced that way.