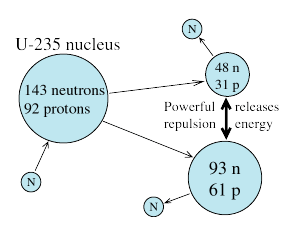
This mode is known to occur spontaneously in artificial elements heavier than uranium. However, the absorption of a neutron by a suitable uranium nucleus--235U or 233U--can also trigger its fission.
A proton aimed at a nucleus, even if headed straight towards it, needs to be accelerated to a considerable energy to overcome the repelling electric force and get close enough to be captured by the strong nuclear force. A neutron, on the other hand, is not repelled and can reach its target, even if it moves quite slowly--e.g. a thermal neutron whose energy is comparable to that of molecules in ordinary matter or in air, about 0.03 eV. Imagine the nucleus as a target of a certain size: then the "nuclear cross section" is the area a projectile must hit to produce a certain reaction (it is also proportional to the likelihood of the projectile "sticking" to the nucleus). Nuclear cross sections are measured in barns, where 1 barn is equivalent to a target size of 10-24 cm2 ("big as a barn" for nuclear physicists). The cross section for a neutron to hit a nucleus varies from one isotope to another, and with the energy of the neutron (similarly for other particles undergoing collision). For instance, the chance of a "thermal" neutron sticking to a nucleus of heavy hydrogen (the 2H isotope or deuterium) is rather small, because that type of hydrogen already has an extra neutron.
As a neutron reaches its target nucleus, one may visualize the nuclear attraction speeding it up, so that it hits with appreciable energy, agitating the target nucleus.
The effects of this extra energy may vary. The target nucleus may simply emit it as a γ ray
photon (end of ref. #11), or it may undergo some internal change--e.g. the neutron may become a proton, emitting an electron (β radioactivity). But with 235U --an isotope forming about 0.7% of natural uranium--the result is usually nuclear fission, splitting the nucleus into two fragments. The products may vary, but typically the ratio of the masses of the two fragments is close to 2:1 .
Nuclear fission was identified in Germany in 1939 by Hahn, Strassman and Lise Meitner. (That was in Nazi Germany--Hahn was awarded the Nobel prize in 1944, while his long-time associate Meitner was Jewish and may have saved her life by escaping to Sweden). Very soon physicists all over realized that the process could provide usable energy. Not only did it release appreciable energy per nucleus, but more important, it also released additional neutrons, making possible a self-sustaining chain reaction.
The Chain Reaction
As already noted, the weak nuclear force tries to adjust the numbers of protons and neutrons in a nucleus to approach equality. However, because protons are positively charged and repel, confining them to the tiny nucleus requires energy, and that shifts stability to a state with extra neutrons. The main isotope of iron has 26 protons and 30 neutrons, making neutrons 53.6% of the total. In uranium 235 (235U) the numbers are 92 and 143, or about 61% of the nucleons are neutrons. The abundance of neutrons in nuclei between these extremes is somewhere in-between, too, increasing with mass. Suppose a U-235 nucleus fissions into isotopes where the neutron fraction in the most stable isotopes is 56%. The distribution in the parent nucleus actually gives them 61%, so that each fragment nucleus has about 4-7 neutrons too many.
Nuclei which have a neutron or two more than their most stable isotope may still be stable. With a greater number of extra neutrons they may adjust by β-radioactivity, emitting an electron as a neutron converts to a proton. Here, however, the imbalance is so great, that a more drastic process occurs: entire neutrons are ejected. When a thermal neutron is captured in 235U, on the average 2.3 neutrons per fission are released, 98% of them "promptly" and 2% delayed by a second or two. These numbers turn out to be quite important.
The Nuclear Reactor
To establish an ongoing chain reaction, slightly more than one neutron per fission is required by a continuing chain reaction. One would expect that with 2.3 neutrons actually generated, we get a generous excess of neutrons. Actually, things are not quite simple (reference 12).
First of all, neutrons which escape from the surface of the uranium fuel are "wasted" to the chain reaction. That means that a "critical mass" is needed for the reaction to proceed. A mass of uranium the size of a peanut has too little depth--too many neutrons escape it without scoring a hit (and the shape of the uranium also may make a difference).
Second, to control the rate of the reaction, it is best to use thermal neutrons. (Nuclear reactors using fast neutrons do exist, but are hard to design and to operate, because all energy is released inside a very small volume, making heat removal a challenge. Fission bombs use fast neutrons.) Fission neutrons start with appreciable energy, and it is necessary to slow them down by repeated collisions in a "moderator" surrounding their source. The ideal moderator is a material not likely to absorb them, with small atoms to maximize the energy transfer: usual choices include "heavy water" D2O --where D is the common notation for deuterium, the heavy isotope of hydrogen (i.e. 2H)-- or very pure carbon, in the form of graphite, the stuff of pencils leads.
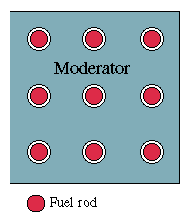
[Figure 4 -- Fuel Rods inside a Neutron Moderator]
The fuel in a typical modern reactor is usually formed into rods (or is contained in hollow rods of stainless steel), which are thrust at appropriate separations into a pool of heavy water (in some designs, ordinary water), or into an array of holes in a core of carbon bricks (drawing). Neutrons released from a fission in one rod soon wander into the moderator and are slowed down there to thermal speed, and after a while (unless they escape or are captured) they reach another rod and initiate another fission event there.
Of course, the extracted energy appears as heat: the enormously fast fission fragments keep colliding with the moderator, and ultimately spread their energy around. If the moderator is water (heavy or "light") it is kept under pressure to raise its temperature, because energy extraction from steam gets more efficient the higher the temperature is. In a solid moderator, pipes carry a fluid to remove the heat by superheated water or by another fluid--even liquid sodium metal has been used (in fast breeder reactors), an extremely tricky substance which bursts into flames if allowed access to air.
Other pipes linked to the heat removal system carry high pressure steam into ordinary steam turbines (similar to those in conventional power stations) which turn electric generators. The cooled-down expanded steam is then turned back to water in cooling towers (often drawn as ominous symbols of nuclear power, though most any steam-driven power station has them) and are recycled to the reactor to pick up more heat.
Third, as fuel is consumed, fission fragments accumulate. These are often fiercely radioactive or "hot" (letting go of 2-3 neutrons makes them more stable, but instability remains) and disposing of them is a major challenge. They may remain "hot" for years and even centuries, and need to be stored out of contact with life and with ground water. Because radioactivity releases energy, initially they also need to be cooled.
Reprocessing and Enrichment
Ideally, before being stored away, a fuel rod needs to be reprocessed. It still contains some useful fuel, which can be reused. Also, some radioactive isotopes produced by fission can be separated and used as radiation sources in medicine or research. Others are pure waste and need to be stored out of contact with the natural environment.
Natural uranium was used in the earliest reactors--but since it is used up rapidly, "enriched uranium" is preferred, in which the fraction of 235U is increased by an "enrichment process." The chemistry of different isotopes is practically the same, so non-chemical separation must be used, with gaseous compounds such as UF6.(uranium hexafluoride). In such a gas, molecules with 235U are about 1% lighter than those with 238U, and therefore at a given temperature they move faster and diffuse more rapidly through porous partitions. Alternatively, a specially designed centrifuge, with a rapidly spinning shaft, may spin the gas and cause heavier molecules to be concentrated in the outer layers.
In either case, because the separated isotopes are so close in mass, the difference in concentration is very small. Therefore uranium separators must be connected in a cascade of many units feeding each other, with the enriched fraction advancing to the next level and the depleted fraction recycled to an earlier one. [Completely depleted uranium is sometimes used for armor-piercing ammunition, since it is very dense and at bullet-speeds packs a lot of kinetic energy.]
Usually most of reactor fuel still consists of the more abundant isotope 238U. Neutron absorption makes this isotope unstable and after some nuclear changes it turns into plutonium 239Pu , an artificial element with 94 protons. Plutonium is also a suitable nuclear fuel, and part of the energy released in a nuclear reactor comes from the fission of plutonium produced there.
Reprocessing nuclear fuel is a difficult task, because it is too dangerous for humans to handle spent fuel directly. All devices involved in reprocessing--including those which pull out used fuel rods and transport them--are operated by remote control, and when discarded many must be stored safely (like the spent fuel) for long periods. One reason partially spent fuel must be removed from reactors and reprocessed is that some fission products absorb neutrons and thus reduce efficiency ("poison the reactor").
Currently the US has stopped reprocessing spent fuel fresh from power stations, and allows it to "cool down" in pools located near reactors, but reprocessing is about to be resumed. France, which gets most of its energy from fission, Russia and other countries do maintain successful reprocessing centers.
Tidbit:
Nuclear reactors were recognized early as ideal power sources for large submarines, since they needed no air and required only infrequent refueling.
Fission reactors were also designed for
powering spacecraft (reference #13). The US launched SNAP 10-A in 1965, but it was shut down after 43 days due to malfunctions. Soviet Russia launched many reactors, which were later detached and boosted to a higher orbit, with a lifetime of centuries. That program ended when the reactor on Cosmos 954, powering an ocean-surveillance radar, failed to detach. The satellite with its reactor crashed on 24 January 1978 into a frozen lake in Canada, creating strong protests and ending the use of reactors in space.
In addition, the radioactive heat produced by plutonium is used in RTGs (Radioisotope Thermal Generators) to power space probes to the outer parts of the solar system, too far from the sun for solar cells to generate sufficient power. RTGs gradually lose power after 20-30 years, and of course they never return to the Earth's neighborhood.
-----------
Nazi Germany also tried to develop nuclear energy during World War II, on a much more limited scale than the Allied powers. However, graphite was regarded as unsuitable, since samples tested for moderator were not pure enough and absorbed too many neutrons. "Heavy water" was chosen instead, a by-product of hydro-electric power stations in Norway, and the Norwegian underground effectively sabotaged its production there.
Problems
(answers in section S-8A-5)
(1) (For this problem, solve first problem (5) in the preceding section)
Assuming a 235U nucleus releases 200 Mev in a fission event (counting some secondary processes; the total averages 215 Mev), how many tons of TNT are needed to obtain the energy yielded by complete fission of 1 gram 238U ?
(2) Compile a glossary, defining briefly in alphabetical order in your own words:
Barn (unit), Cascade for isotope enrichment, Chain reaction (nuclear), Critical mass,
Cross section (for nuclear interaction), Delayed neutrons, Enrichment (of uranium),
Fission (nuclear), Fission fragments, Fuel rods, Graphite, Heavy water,
Isotope separation by centrifuges, Isotope separation by porous partitions, Photon,
Plutonium, "Poisoning" of a nuclear reactor, Prompt neutrons, Reprocessing of nuclear fuel, Thermal neutron.
|