|
Optional: Doppler Effect and Dark Matter ------------------------ Optional: Quantum Physics Hot Objects and Bohr's Model Bohr's Model -------------------------- our Galactic Center of Atoms and Nuclei |
The reader may by now appreciate how fundamental Einstein's formula is
It was discovered in 1905, the same year in which Einstein published his famous article on relativity--and yet his 1921 Nobel Prize was awarded, not for relativity but for the above equation. The thing to note is that the constant h, which appears here and is central to quantum theory, was already known in 1905. We call it Planck's constant because it was first proposed by Max Planck in 1900.
If energy is measured in joules and frequency in oscillations-per-second, the "dimensions" of h--the way it is related to the units of physical quantities--is joules times seconds (frequency is 1/sec, "oscillations per second"). It turns out to be a very small number
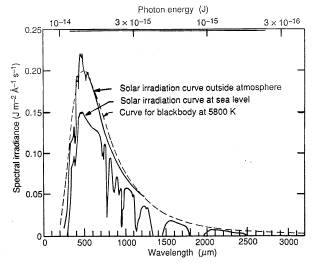
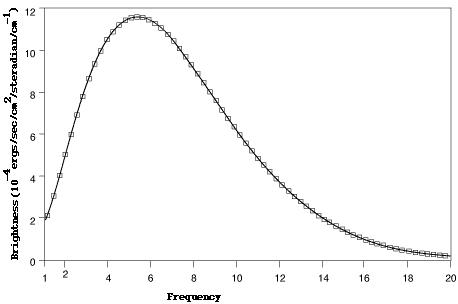
and this smallness is tied to the fact that "classical physics" of the laws of Newton and Maxwell holds fairly well as long as energies and times give a product significantly larger than h. Only on the atomic scale do the new laws dominate. The rest of this section describes what Max Planck did and is not essential to other sections here. He was not concerned with the "line spectra" of narrowly defined wavelengths (and frequencies), as emitted by individual atoms in a hot gas. Instead he wanted to explain the continuous spectrum emitted by hot solids--also known as "black body spectrum" because it is supposedly emitted by an ideal hot object ("black body") whose structure gives no special preference to any color (as happens with green grass, blue jeans and red bricks). From everyday experience we know that heated solid objects emit light, and as a their temperature increases, their dominant color moves increasingly towards the blue end of the spectrum. A warm hand emits infra-red light--invisible to the eye but detectable by organs on some snakes (also by sensors on heat-seeking anti-aircraft missiles). A blacksmith handling hot iron makes it glow a dull red, then if the coals are fanned and the temperature increases, orange. The filament of a lightbulb fed by a fading battery also glows orange, while a fresh battery makes the lightbulb glow yellow-white.
|